INVESTIGATIONS ON DOMINANT GLUCOSINOLATES IN RAPESEED GERMPLASM COLLECTED IN CHINA*
Li Peiwu1, Li Guangming1, Zhang Wen1, Yang Mei1 and Zeng Jianqiang2
1Oil Crops Research Institute, Chinese Academy of Agricultural Sciences, Xu Dong 2nd Rd, Wuchang, Wuhan, Hubei 430062, P. R. China, e-mail: ocgil@public.wh.hb.cn
2Oil Crops Division of Agriculture Bureau, Ministry of Agriculture of P. R. China
ABSTRACT
Dominant individual glucosinolates in 133 accessions of B. napus, 60 accessions of B. juncea and 170 accessions of B. campestris of rapeseed germplasm collected in China were studied quantitatively by high performance capillary electrophoresis (HPCE) method based on micellar electrokinetic capillary chromatography (MECC) using cetyltrimethylammonium bromide (CTAB). Progoitrin and gluconapin were found to be the dominating glucosinolates in B. napus germplasm while 4-hydroxyglucobrassicin, gluconapoleiferin, gluconasturtin and glucobrassicin were present in small amount. B. juncea germplasm was characterized by the predominance of sinigrin and gluconapin. Sinigrin concentration varied from 50% to 90% of the total glucosinolates depending on the region where the accessions were collected. Most high sinigrin materials were from Yunnan and Guizhou production area and lower sinigrin ones from Xing Jiang, Qinghai and Tibet. Gluconapin and 4-hydroxyglucobrassicin accounted for more than 80% of the total glucosinolates content in B. campestris accessions. Progoitrin, sinigrin, gluconapoleiferin, glucobrassicin, neoglucobrassicin, glucotropaeolin and gluconasturtin were the minor ones. The study appeared to provide practical information for both rapeseed genebank establishment and double low rapeseed breeding.
KEYWORDS: progoitrin, gluconapin, sinigrin, HPCE-MECC, rapeseed resources, China
INTRODUCTION
Rapeseed is the most important oil crop with annual 6,8 million ha production area and 9,5 million metric tons of total yield in China where it has been cultivated for more than 2000 years (Hougen and Stefansson, 1983; Houli, L., 1987). Nowadays, 65% consumption of edible plant oil in China is from rapeseed and canola oil. Yangzi Rever valley has been the most productive area where rapeseed production accounts for 70 - 80% of total rapeseed yield in recent years. China is a country rich in rapeseed germplasm resources due to the long history of both spring and winter rapeseed cultivation and multi-rotation systems. Xingjiang wild rape was discovered and identified (Zhaomu, W., 1995, Li S. et al., 1995). Yunnan and Guizhou were proposed as the original center for B. juncea and Anhui might be the original center for B. campestris in China based on the geographical frequency distribution of erucic acid gene polymorphism (Hanzhong, W., et al., 1995). Rapeseed germplasm collection and evaluation in China started in 1970s. About 5500 accessions have been collected and evaluated agronomically (Xiuzhen, Q. et al., 1992; Hancheng, X. and Hong, X., 1993). Some elite accessions for example Zhong RS-1 which was high resistant to sclerotinia sclerotiorum, Tibet Qiongguo with oil content up to 52,7% were discovered and used in breeding programme, which resulted in releasing a series of rapeseed varieties. Although double
*A project supported by NSFC with project No. 39770452 from 1998 to 2000
low rapeseed breeding started in early 1980s in China, little work has been done on glucosinolates composition in germplasm resources evaluation.
The strong interests in glucosinolates were from their various flavour, off-flavour, toxic and antinutritional effects as well as the positive physiological effects such as anticarcinogenesis caused by glucosinolates and their degradation products (Bjergegaard, C. et al., 1994; Feldl.C. et al., 1994; Loft. S. et al., 1992; Young, T. B. and Wolf. D. A., 1988; Wattenberg, L. W. et al., 1986). It was reported that individual glucosinolate and total glucosinolates in oilseed rape varied in inheritance (Lethenborg, P. et al., 1995, Getinet, A. et al., 1995). Indolyl glucosinolates was found to be closely related to rapeseed meal quality (Jensen, S.K. et al., 1991). Therefore, the antinutritional and positive physiological effects varied with different kinds of glucosinolates. However, available information on glucosinolates composition in rapeseed germplasm collected in China is still fragmentary.
In order to establish rapeseed genebank and to further evaluate rapeseed germplasm resources for better use of the materials in rapeseed breeding, dominant individual glucosinolates in rapeseed germplasm resources of China were studied.
MATERIALS AND METHOD
133 accessions of B. napus used in the study were collected from Sichuan (2 acc.), Anhui (6 acc.), Zhejiang including Shanghai (54 acc.), Jiangsu (20 acc.), Qinghai (6 acc.), Xingjiang (1 acc.), Jiangxi (8 acc.) and Hubei (36 acc.) provinces. 60 accessions of B. juncea were from Sichuan (21 acc.), Tibet (15 acc.), Xingjiang (14 acc.), Guizhou (5 acc.) and Yunnan (5 acc.) provinces. 170 accessions of B. campestris were from Tibet (4 acc.), Guizhou (132 acc.), Qinghai (8 acc.), Yunnan (14 acc.), Anhui (9 acc.) and Hubei (3 acc.). After agronomic traits were evaluated in field all the resource materials were conserved in both Beijing National Long-term Crops Genebank and Wuhan Middle-long-term Oil Crops Genebank located in Oil Crops Research Institute of Chinese Academy of Agricultural Sciences. Intact individual glucosinolates in seed were analysed by high performance capillary electrophoresis method based on micellar electrokinetic capillary chromatography using cetyltrimethylammonium bromide as described elsewhere (Peiwu Li et al., 1994).
RESULTS AND DISCUSSIONS
Dominant glucosinolates in rapeseed germplasm
Mean percentage of individual glucosinolates was caculated based on total glucosinolates concentration and showed in Figure 1. Progoitrin and gluconapin were the predominant ones in all 133 accessions of B. napus resources including 53 accessions of double low materials and 80 conventional double high landraces although the total glucosinolates contents varied from 10,2 to 95,5 mmol/g×seed. The other glucosinolates including 4-hydroxyglucobrassicin, gluconapoleiferin, gluconasturtin and glucobrassicin were the minor ones.
sinigrin was the most dominating glucosinolate in B. juncea resources. However, the 60 accessions analyzed were classified into two groups according to the predominance of sinigrin. The first one was the 42 accessions from Yunnan and Guizhou where might be the original center for B. juncea (Hanzhong, W. et al., 1995). These materials possessed more than 87% sinigrin in total glucosinolates as the only dominant glucosinolate (Figure 1). The other were those accessions characterized by approximately 50% sinigrin and 40% gluconapin, which were collected from Xingjiang, Tibet and Sichuan provinces. It seemed that geographical and ecological difference affected sinigrin predominance in B. juncea germplasm.
In all 170 B. campestris accessions, gluconapin was the most dominant glucosinolates. Similar results were obtained by Jixiang H. (1995) with yellow-seeded rapeseed embryo. It was also found that 4-hydoxyglucobrassicin in B. campestris existed in much higher amount than that in B. napus and B. juncea materials although the percentage was only 8% of the total glucosinolates. A landrace accession Duishuiqiongbai with 18 mmol/g×seed total indolyl glucosinolates was identified. This provided a hint for screening high indolyl glucosinolates materials in the related project in our laboratory.
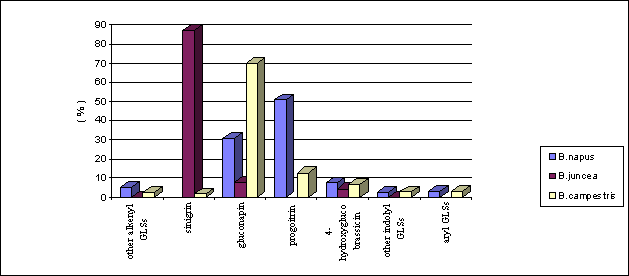
Figure 1. Mean percentage of dominant glucosinolates in rapeseed germplasm resources collected in China
Total glucosinolates contents
Total intact glucosinolates contents varied with accessions and collection origins (Table 1). The variation was from 10,2 mmol/g×seed to 95,5 mmol /g×seed in B. napus, 20,9 to 88,9 mmol/g×seed in B. juncea and 29,5 to 123,6 mmol/g×seed in B. campestris. The abundant diversity of glucosinolate contents in rapeseed germplasm resources provided more choice not only for oilseed rape breeding but also for special quality improvement such as vegetable and condiment rapeseed breeding (Quiros, C. F.,1995).
Table 1. Total glucosinolates concentration in rapeseed germplasm resources of China (mmol/g ×seed)
|
origin |
Tibet |
Xingjiang |
Qinghai |
Guizhou |
Yunnan |
Sichuan |
Hubei |
Jiangxi |
Anhui |
Jiangsu |
Zhejiang |
|
B. napus (double low) |
|
25,2 |
29,1 ±7,5 |
|
|
24,5 ±0,6 |
15,5 ±12,7 |
|
33,5 ±11,2 |
25,8 ±15,4 |
15,2 ±8,2 |
|
B. napus |
|
|
64,3 ±16,7 |
|
|
|
61,8 ±12,5 |
62,7 ±17,0 |
|
56,0 ±16,2 |
69,1 ±16,1 |
|
B. juncea |
63,0 ±12,5 |
30,1 ±17,6 |
|
68,2 ±15,7 |
65,0 ±15,5 |
58,7 ±17,4 |
|
|
|
|
|
|
B. campestris |
95,0 ±14,6 |
|
77,8 ±23,7 |
74,9 ±20,2 |
44,7 ±12,0 |
|
95,0 ±23,1 |
|
80,9 ±10,8 |
|
|
CONCLUSION
Dominant glucosinolates in rapeseed germplasm collected in China were progoitrin and gluconapin in B. napus resources, sinigrin and gluconapin in B. juncea and gluconapin in B. campestris materials. The rich diversity of glucosinolates profiles and contents in rapeseed resources provides more choices for exploitation of the germplasm in rapeseed breeding.
ACKNOWLEDGEMENTS
Natural Science Foundation of China (NSFC) is greatly acknowledged for the financial support. The authors are grateful to Prof. Wu Xingyong and Li Yunchang for their technical assistance. Thanks are also given to associate Prof. Wu Xiaoming for providing some of the rapeseed germplasm materials.
REFERENCES
Feldl, C. et al., 1994. GCIRC Bulletin. 10: 128-133.
Getinet, A. et al., 1995. Proceedings of the 9th International GCIRC Rapeseed Congress, Cambridge, UK, Vol. 4:1098-1100.
Hancheng X. and Hong X., 1993. Oil Crops of China. No.3: 12-14.
Hanzhong W., 1995. Proceedings of the 9th International GCIRC Rapeseed Congress, Cambridge, UK, Vol. 4:1076-1078.
Hougen, F. W., and Stefansson, B. R. 1983. Rapeseed, in Advances in Cereal Science and Technology. Vol 5. St. Paul: American Association of Cereal Chemists. 261-289.
Houli L.,1987. Practical Rapeseed Cultivation. Shanghai Science and Technology Press of China. 6-7; 32-36.
Jensen, S. K. et al., 1991. Proceedings of the 8th International GCIRC Rapeseed Congress, Saskatoon, Canada, 1359-1361.
Jixiang, H. et al., 1995. Proceedings of the 9th International GCIRC Rapeseed Congress, Cambridge, UK, Vol. 4:1134-1136.
Li Sun and Chunyun G., 1995. Proceedings of the 9th International GCIRC Rapeseed Congress, Cambridge, UK, Vol. 4:1083-1085.
Loft, S. et al., 1992. Food Chem. Toxicol. 30: 927-935.
Peiwu, Li et al., 1994. GCIRC Bulletin. 10:155-160.
Quiros, C. F., 1995. Proceedings of the 9th International GCIRC Rapeseed Congress, Cambridge, UK, Vol. 4:1057-1062.
Wattenberg, L.W. et al., 1986. Diet, Nutrition, and Cancer. Japan Scientific Society Press, 193.
Xiuzhen Q. et al., 1992. Oil Crops of China. No.3:38-42.
Young, T.B. and Wolf, D.A., 1988. International Journal of Cancer. 42:167-175.
Zhaomu W., 1995. Proceedings of the 9th International GCIRC Rapeseed Congress, Cambridge, UK, Vol. 4:1079-1082.