FISH-ing for new rapeseed lines: the application of molecular cytogenetic techniques to Brassica breeding
R.J. Snowdon1, A. Köhler2, W. Köhler1and W. Friedt1
1 Institute for Crop Science and Plant Breeding, Justus-Liebig University Giessen, Ludwigstr. 23/27, D-35390 Giessen, Germany
2 Institute for Human Genetics, Justus-Liebig University Giessen,
Schlangenzahl 14, D-35392 Giessen, Germany
ABSTRACT
Fluorescence in situ hybridisation (FISH) techniques, which enable the direct chromosomal localisation of labelled DNA probes, have been increasingly applied to plant genome mapping in recent years. We have developed FISH methods for the accurate localisation of repetitive DNA sequences at chromosomal sub-arm level in Brassica species. In addition we apply genomic in situ hybridisation (GISH) for identification and characterisation of parental genome components in rapeseed hybrids. The detection of short, low-copy molecular markers is not possible by FISH, however this shortcoming could be overcome by physical localisation of megabase DNA clones containing markers of interest. High-resolution FISH can provide information about ordering and physical distances between molecular markers, both important considerations for physical mapping and positional cloning. Practical applications of FISH and GISH in rapeseed breeding are discussed.
KEYWORDS: fluorescence in situ hybridisation, FISH, GISH, hybrids
INTRODUCTION
Knowledge of the physical organisation of DNA sequences within the genome is critical for the understanding of genome structure and function. The technique of fluorescence in situ hybridisation (FISH) allows the precise physical localisation of genes or DNA sequences on cytological preparations. In recent years FISH has enabled enormous progress in studies of genome organisation in humans and other mammals, and coupled with rapid technological advances it is being increasingly adapted for the localisation and characterisation of various classes of DNA sequences in plant genomes. Applications of FISH methods in plant genome mapping have been reviewed in detail by Jiang and Gill (1994, 1996).
The use of total genomic DNA as a FISH probe (genomic in situ hybridisation, or GISH) is especially useful for diagnostic studies of the amount and integration of foreign chromatin in interspecific and intergeneric plant hybrids (see Heslop-Harrison and Schwarzacher 1996). Hybrids between high-yielding rapeseed cultivars and related species are relatively easily produced and often used to develop new lines containing desired traits like pest or disease resistance. Great progress in rapeseed breeding has resulted from the application of in vitro techniques for the generation of viable offspring from interspecific and intergeneric hybrids (Friedt and Lühs 1998). Here we describe the application of methods for GISH analysis of intergeneric Brassica hybrids (Snowdon et al. 1997b) for the characterisation of backcross progeny from B. napus hybrids exhibiting nematode resistance introduced from Raphanus sativus (Voss et al. in press), and Phoma resistance from Sinapis arvensis and Coincya monensis respectively.
The use of more specific DNA sequences as FISH probes enables their localisation to discrete chromosomes or chromosomal regions. Localisation of repetitive DNA probes gives important information on the distribution of repetitive sequence motifs throughout the genome (Heslop-Harrison et al. 1997, Schmidt and Heslop-Harrison 1998), an important aspect in the genetic mapping of molecular markers. Physical information about the extent and localisation of both repetitive and low-copy sequences can be compared with genetic maps of molecular markers, allowing associations to be made between distinct chromosomal regions and molecular marker linkage groups. Additionally, hybridisation patterns of repetitive DNA sequences can be used as chromosome markers in plants like rapeseed which have numerous small chromosomes with few cytological features to allow their identification using traditional cytogenetic methods.
The heavily condensed chromatin characteristic of plant chromosomes prevents the reliable localisation of single- or low-copy sequences shorter than 10kb on plant metaphase chromosomes (Jiang and Gill 1994, Fuchs et al. 1996). The development of megabase-DNA (BAC, YAC) libraries, however, provides a way to overcome this limitation: Low-copy BAC or YAC clones can now be localised directly onto metaphase chromosomes or in interphase nuclei. This development has important implications for the integration of molecular marker linkage maps with physical information, since simultaneous hybridisation of genetically linked clone sequences can give important information about the spatial distribution of molecular markers along chromosome arms. Using multicolour FISH to extended chromatin fibres it is now also possible to estimate physical distances among genetically linked megabase-DNA fragments containing genes or markers of interest (Jackson et al. 1998). Such physical data is extremely useful for positional cloning strategies.
We have developed methods for the reliable chromosomal localisation of FISH signals in Brassica species and a GISH technique that allows identification of alien chromatin in intergenomic rapeseed hybrids. Use of FISH with repetitive probes for the identification of chromosomes is discussed, along with the practical application of GISH techniques for the characterisation of various rapeseed hybrids containing genes of interest. Future aims include the application of fibre-FISH to obtain physical information about spatial relationships among molecular markers.
MATERIAL AND METHODS
Mitotic metaphases were generated from seedlings of diverse Brassica cultivars for FISH and from hybrid plants (see Table 1) for GISH. Cytological preparations were made from young root tips using a modification of the droplet method of Schwarzacher et al. (1994). Briefly, whole seedlings or excised roots were incubated in 2 mM 8-hydroxyquinoline for 90 min at room temperature and a further 90 min at 4°C before fixation in ethanol-acetic acid (3:1). After digestion in cellulase and pectinase, root tips were subjected to 30 min hypotonic treatment in 75 mM KCl, then washed for 20 min in 60% acetic acid to clear cytoplasm before being suspended in ethanol-acetic acid (3:1) and spread on cleaned slides at -20°C. Fibre-FISH preparations were generated from B. oleracea L. (white cabbage cv. ´Braunschweiger´) following the method of Jackson et al. (1998).
Table 1: Intergeneric hybrids analysed using GISH
|
Target genome |
Donor |
Resistance character |
Backcrossed with |
|
B. napus cv. ´Drakkar´ |
Raphanus sativus |
Beet cyst nematode resistance |
B. napus cv. ´Lisandra´ |
|
B. napus cv. ´Madora´ |
Sinapis arvensis* |
Phoma resistance |
B. napus cv. ´Ceres´ |
|
B. napus cv. ´Loras´ |
Coincya monensis* |
Phoma resistance |
B. napus cv. ´Loras´ |
* Sinapis and Coincya crosses were kindly provided by Prof. Maria Dolores Sacristán, FU-Berlin, Germany
In situ hybridisation followed methods described previously (Snowdon et al. 1997b), with slight modifications. For multicolour FISH, a 25S rDNA clone from Arabidopsis thaliana and the 5S rDNA subunit from Beta vulgaris were labelled by nick translation with the fluorochromes Cy3 and fluorescein, respectively. For GISH probes, genomic DNA extracted from Raphanus sativus, Sinapis arvensis and Coincya monensis was directly labelled with Cy3 and mixed with a 50-fold volume of unlabelled, sheared B. napus genomic DNA. GISH probes were preannealed for 20 min at 37°C before hybridisation. All slides were washed at 42°C for 5 min in 2x SSC and 10 min in 0.2x SSC before being counterstained with DAPI. Composite fluorescence images were obtained using a Leica DMR fluorescence microscope fitted with specific single-band filters for DAPI, FITC and Cy3 and an integrating black and white CCD camera driven by Leica QFISH software.
RESULTS AND DISCUSSION
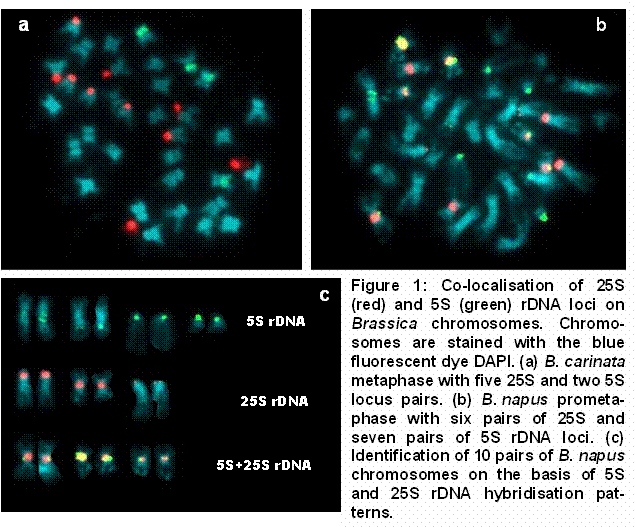
Multicolour FISH was used for the co-localisation of 25S and 5S rDNA loci on mitotic metaphase and prometaphase chromosomes from various Brassica species (e.g. Figure 1a). In B. napus, co-localisation of the six 25S rDNA loci (Maluszynska and Heslop-Harrison 1993, Snowdon et al. 1997a) with the seven 5S rDNA loci (Figure 1b) enables the reliable identification of 10 chromosome pairs (Figure 1c). Using conventional cytogenetic methods the identification of rapeseed chromosomes is often difficult or impossible. FISH with rDNA and other repetitive DNA probes provides molecular cytogenetic markers for accurate chromosome identification, opening the possibility for a correlation of molecular marker linkage groups with individual chromosomes.
|
|
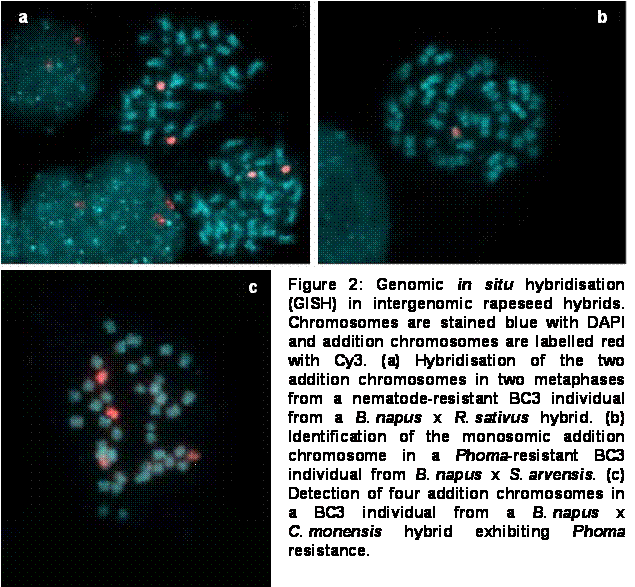
Using GISH, backcross offspring from three different intergeneric B. napus hybrid crosses could be effectively characterised. Figure 2 shows the identification with GISH of addition chromosomes in BC3 individuals from B. napus crosses with Raphanus sativus, Sinapis arvensis and Coincya monensis, respectively. In both the R. sativus and S. arvensis crosses, fertile BC3 individuals exhibiting the desired resistance character were found which contained monosomic addition chromosomes from the respective donor genome. GISH is now being used for the characterisation of resistant BC4 offspring.
|
|
The ultimate aim for each of the hybrids described above is a high-quality rapeseed cultivar containing the desired resistance genes on a stable chromosome introgression. Although intergenomic recombination appears to be low in the hybrids described here, it is hoped that useful introgressions will be present in resistant BC4 plants. That donor-genome introgressions can potentially be observed by GISH was confirmed by the discovery of a small translocation (along with two complete addition chromosomes) in a non-resistant BC3 B. napus x R. sativus individual. The visualisation of small translocations in rapeseed hybrids by GISH can be problematic, however, because the chromosome arms of Brassica and closely related species contain few of the dispersed repetitive sequences that contribute to GISH signals (Heslop-Harrison and Schwarzacher 1996). This is not necessarily a major problem, however, because in eventual late-backcross introgression lines it will be the plant performance rather than the quantity of donor chromatin which is the deciding factor in the selection of breeding material.
A fibre-FISH technique for the high-resolution localisation of FISH signals on extended chromatin fibres is presently being adapted for use in Brassica species. Until now only repetitive probes have been hybridised. With further improvements in the technique, however, it is hoped that low-copy markers will also be able to be reliably detected. As described by Jackson et al. (1998), this would open the possibility to compare physical distances between linked molecular markers with the corresponding genetic distances. Such information will be extremely useful for future physical mapping and positional cloning efforts.
ACKNOWLEDGEMENTS
The authors thank Axel Voss for his work in developing and testing the Raphanus sativus hybrid material used in this study. The Sinapis arvensis and Coincya monensis hybrids were kindly provided by Hendrik Winter and Prof. Maria Dolores Sacristán, FU-Berlin, Germany. This work was financed by a grant from the Deutsche Forschungsgemeinschaft (DFG: Ko701-15/1).
REFERENCES
Friedt W. and W.W. Lühs (1998) Recent developments and perspectives of industrial rapeseed breeding. Fat-Lipid 100: 219-226
Fuchs J., D.U. Kloos, M.W. Ganal and I. Schubert (1996) In situ localization of yeast artificial chromosome sequences on tomato and potato metaphase chromosomes. Chrom. Res. 4: 277-281
Heslop-Harrison J.S. and T. Schwarzacher (1996) Genomic southern and in situ hybridization for plant genome analysis. In: Jauhar P.P. (ed.) Methods of genome analysis in plants. CRC Press Inc., Boca Raton, Florida. pp. 163-179
Heslop-Harrison J.S., A. Brandes, S. Taketa, T. Schmidt, A.V. Vershinin, E.G. Alkhimova, A. Kamm, R.L. Doudrick, T. Schwarzacher, A. Katsiotis, S. Kubis, A. Kumar, S.R. Pearce, A.J. Flavell and G.E. Harrison (1997) The chromosomal distributions of Ty1-copia group retrotransposable elements in higher plants and their implications for genome evolution. Genetica 100: 197-204
Jackson S.A., M.L. Wang, H.W. Goodman and J. Jiang (1998) Application of fiber-FISH in physical mapping of Arabidopsis thaliana. Genome 41: 566-572
Jiang J. and B.S. Gill (1994) Nonisotopic in situ hybridization and plant genome mapping: the first ten years. Genome 37: 717-725
Jiang J. and B.S. Gill (1996) Current status and potential of fluorescence in situ hybridization in plant genome mapping. In: Patterson A.H. (ed.) Genome mapping in plants. Academic Press, San Diego, California. pp. 127-135
Schmidt T., and J.S. Heslop-Harrison (1998) Genomes, genes and junk: the large-scale organization of plant chromosomes. Trends in Plant Science 3: 195-199
Schwarzacher T., A.R. Leitch and J.S. Heslop-Harrison (1994) DNA:DNA in situ hybridization - methods for light microscopy. In: Harris N. and Oparka K.J. (eds) Plant Cell Biology: A practical approach. Oxford University Press, Oxford. pp 127-155
Snowdon R.J., W. Köhler and A. Köhler (1997a) Identification and characterization of rDNA loci in the Brassica A and C genomes. Genome 40: 582-587
Snowdon R.J., W. Köhler, W. Friedt and A. Köhler (1997b) Genomic in situ hybridization in Brassica amphidiploids and interspecific hybrids. Theor. Appl. Genet. 95: 1320-1324
Voss, A., W.W. Lühs, R.J. Snowdon and W. Friedt (in press). Development and molecular characterization of nematode-resistant rapeseed (Brassica napus L.). Genetics and Breeding for Crop Quality and Resistance, Proc. XV Eucarpia General Congress, September 20-25th, 1998, Viterbo, Italy. Kluwer Academic Publ., Dordrecht, Netherlands.